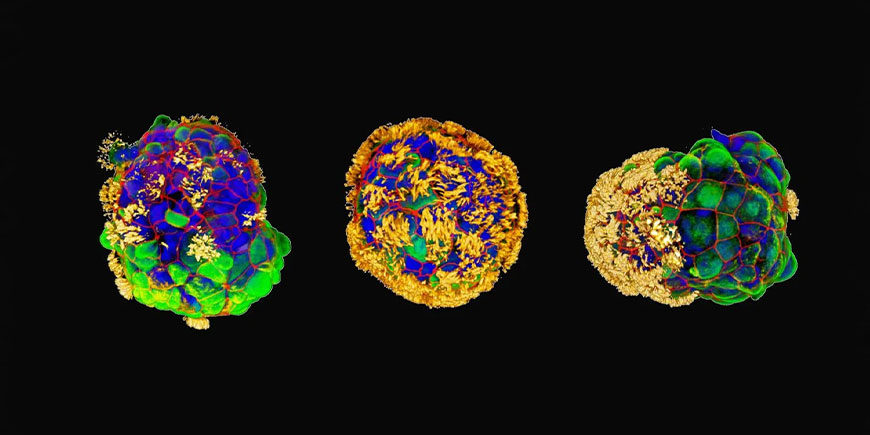
Anthrobots are small human cell biorobots, distinguished by their ability to repair damaged nerve tissue.
Biologists at Tufts University and Harvard University’s Wyss Institute have created robotic entities called “anthrobots” from human cells. These multicellular structures are not only able to move autonomously in liquid thanks to protein cilia, but have also demonstrated an astonishing ability to promote wound healing in other human tissues.
An innovation that opens up new avenues in regenerative medicine and tissue repair, with potentially revolutionary applications.
Results are published in Advanced Science.
The Anthrobot genesis
Anthrobots were developed by a team of researchers led by biologists Michael Levin and Gizem Gumuskaya.
The basic concept is not new: in 2020, researchers had already successfully experimented with the creation of ‘xenobots‘ using frog embryonic cells. The experiment not only raised scientific questions, but also opened up the debate on ethical and social issues, such as the definition of life itself, or whether scientists have the right to ‘play God’ in the laboratory.
Later, Levin and Gumuskaya turned their attention to human cells, in particular those of the windpipe. These cells have a peculiar cilia-like structure and the ability to move autonomously. Their natural function of expelling particles trapped in the airways was exploited to assemble them into larger structures to reconstruct parts of tissue damaged by disease or accident.
Interestingly, the researchers achieved this without altering the cells’ DNA, but rather by biologically reprogramming them to create new structures and tissues.
Regenerative medicine potential
What distinguishes the anthrobots from other similar experiments is their apparent ability to induce healing in other tissues during their two-month ‘lifespan’ (the maximum achieved so far).
When the researchers made the anthrobots ‘walk’ on a flat layer of human neurons grown in a plate that had been damaged by a scratch, they found that the empty space helped the neurons to regenerate.
The mechanism by which the robots trigger the healing process is not yet clear. However, we do know that they do not form a simple mechanical bridge between the two edges of the wound, as small pieces of an inert polysaccharide gel do not have the same effect.

The results, although preliminary, open up the possibility of using these robots to influence the behaviour of other cells. For example, one of the next steps will be to test whether the curative effect is maintained in brain organoid models that mimic a neurodegenerative disease.
In addition to neural healing, researchers see potential applications in the treatment of atherosclerosis, repair of spinal cord or retinal nerve damage, detection of pathogens such as bacteria or cancer cells, and even targeted drug delivery.
Disputes and the future
Not everyone in the scientific community is convinced of the value of these discoveries. Some researchers, such as Jamie Davies of the University of Edinburgh, remain sceptical about defining these aggregates as ‘robots’. However, the undeniable biological functionality demonstrated by anthrobots, particularly in their effect on damaged neural tissue, suggests unexplored potential.
In summary, anthrobots are an excellent example of how the frontiers of biology, technology and medicine are overlapping in increasingly innovative and surprising ways. If their healing and regenerative abilities are confirmed and further developed, we could witness a true revolution in wound care and tissue regeneration, opening up new horizons in the medicine of the future.